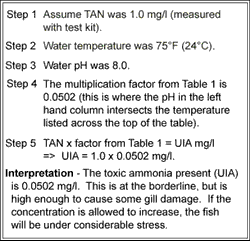
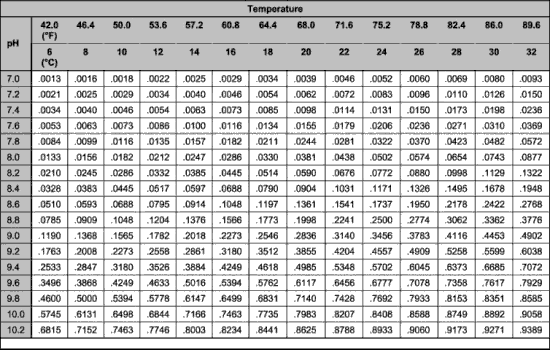
11 This method covers the determination of ammonia in drinking ground surface and saline waters domestic and industrial wastes. Nitrate nitrite and organic nitrogen.
Clean the prepared sample cell.
. The experiment was done by using the blank sample and wastewater sample from lake. The analysis starts with an acid digestion of the sample organics converting organic nitrogen to ammonia. 1USEPA accepted for wastewater analysis distillation required Method 3502.
Add 7 drops salicylate ammonia 2 3979. This was to allow the powder to properly mix with the water sample. The key difference between ammonia and ammoniacal nitrogen is that the ammonia is an inorganic compound having the chemical formula NH3 whereas the ammoniacal nitrogen is a measure of the amount of ammonia in a sample.
National Institutes of Health. A certain mass of NH 4 Cl double of its mass of Ca OH 2 is taken in a boiling tube. Insert the blank into the cell holder.
As specified on the SDS. These methods include spectrophotometric. After adding ISA the solution should have a pale blue color indicating the pH is 11.
It was necessary because of the dilution incident to the addition of the sulphuric acid to Sample IV to compute the results obtained for ammonia nitro- gen in terms of the original urine. Higher concentrations can be determined following dilution. The speed of this reaction is increased by increasing the digestion temperature to 395 C.
LaMotte Ammonia-Nitrogen Test Kit Code 3304 Gloves and goggles Waste container Procedure Ammonia Put on gloves and goggles. The 30 seconds-timer-button was clicked on the spectrophotometer. Description of the process.
Please Contact Genprice for SDS. Insert the prepared sample into the cell holder. In general around 6 to 24 mgdL 21 to 85 mmolL is considered normal.
One molecule of nitrogen gas N2 and three molecules of hydrogen gas H2 are combined to yield two molecules of ammonia NH3. Ammonia nitrogen refers to the nitrogen existed in terms of ammonium cation which is ionized and formed by the protonation of ammonia NH3 and the dissolved ammonia NH3-N. 8600 Rockville Pike Bethesda MD 20894 USA.
Higher concentrations can be determined by sample dilution. N2 3H2 2NH3. Distillation is required for wastewater and seawater.
Here we review studies that focus on analytical methods for determining ammonia nitrogen in natural waters that were published between 2014 to mid-2019. Ammonia is a nitrogen waste compound that is normally excreted in the urine. DETERMINATION OF AMMONIA NITROGEN BY SEMI-AUTOMATED COLORIMETRY 10 SCOPE AND APPLICATION.
As specified on the SDS. As for the prepared sample preparation NitriVer 3 Nitrite Reagent Powder Pillow was added into the sample cell. Fill one of the plastic test tubes 0124 to the 5 ml line with sample water from the creek.
National Library of Medicine. The reported lower range is. The total nitrogen per cc.
Ammonia is a colorless gas with a strong pungent odor. Depending on the Concentration. Please Contact Genprice for Certificate of Analysis.
Nitrogen AmmoniaDOC3165301078 USEPA1Nessler Method2Method 8038 002 to 250 mgL NH3N Reagent Solution Scope and application. As specified on COA. This requires boiling the sample in concentrated sulfuric acid potassium sulfate and a copper catalyst to convert the organic nitrogen to ammonia.
Of urine was determined in all four samples using the Kjeldahl method. National Center for Biotechnology Information. A delivery tube is fitted to the mouth of the boiling tube using a cork and the boiling tube is.
Results show in mgL NH3N. As specified on the COA. One milligram per liter of ammonia-nitrogen is equivalent to 122 mgL of ammonia.
Ammonia nitrogen in the range from 05 to 1000 mg NH 3 NL directly in reagent and effluent waters. Add 2 mL of ammonia ISA to the standard. The iron catalyst is used to help fixate the reaction since the reaction is reversible and all the reactants cannot be fully converted to ammoniashow more content Zumdahl S.
Specific Gravity at 25. Add 10 drops salicylate ammonia 1 3978. Confusion on this can result in reporting an ammonia violation when in fact there may not be a violation at all.
The next article will deal with the other forms of nitrogen that we are concerned with in the wastewater field. The display shows 000 mgL NH3N. For water wastewater and seawater.
The blank sample was used to callibrate the equipment which the reading is 000mgL which means the reading was accurate. Although it is useful on many occasions it is a toxic. The yellow colour or reddish brown colour typical of ammonia N can be measured in a spectrophotometer in the wavelength of 400u0013 500 nm with a light path of 1cm.
Results of the BUN test are measured in milligrams per deciliter mgdL in the United States and in millimoles per liter mmolL internationally. An elevated level of ammonia in the blood is an excessive accumulation of ammonia in the blood. Ammonia is a gaseous compound with a characteristic pungent odor.
Nitrogen comes in several forms which are ammonia nitrate nitrite and organic nitrogen. Apparatus Spectrophotometer or Nessler tube tall form 50 mL or 100 mL capacity pH meter Reagents click to check the preparation of reagents Zinc sulphate solution. When the timer expires clean the blank sample cell.
Allow the solution to stir for 1 minute and begin calibration. An elevated level of ammonia in the blood occurs when the kidneys or liver are not working properly allowing wastes to remain in the bloodstream. Department of Health and Human Services.
Calibration is time sensitive as ammonia in the sample is no longer sufficient 4 minutes after ISA is added. But normal ranges may vary depending on the reference range used by the lab and your age. Ammonia nitrogen Lab Grade from Genprice.
12 The applicable range is 001-20 mgL NH. Ammonia gas is generally prepared in the laboratory by quietly heating ammonium chloride NH 4 Cl and slaked lime Ca OH 2. The sample cell was capped and shaken gently during the 30 seconds-timer.
1 Introduction 11 Background of Ammonia Ammonia as one of the major contaminant from both municipal and industrial waste water is widely found in aquatic systems.

Nitrogen Cycle Accessscience From Mcgraw Hill Education

Oc Ocean Water And Current Computer Lab

Fraction Of Un Ionized Ammonia In Aqueous Solution At Different Ph Download Scientific Diagram
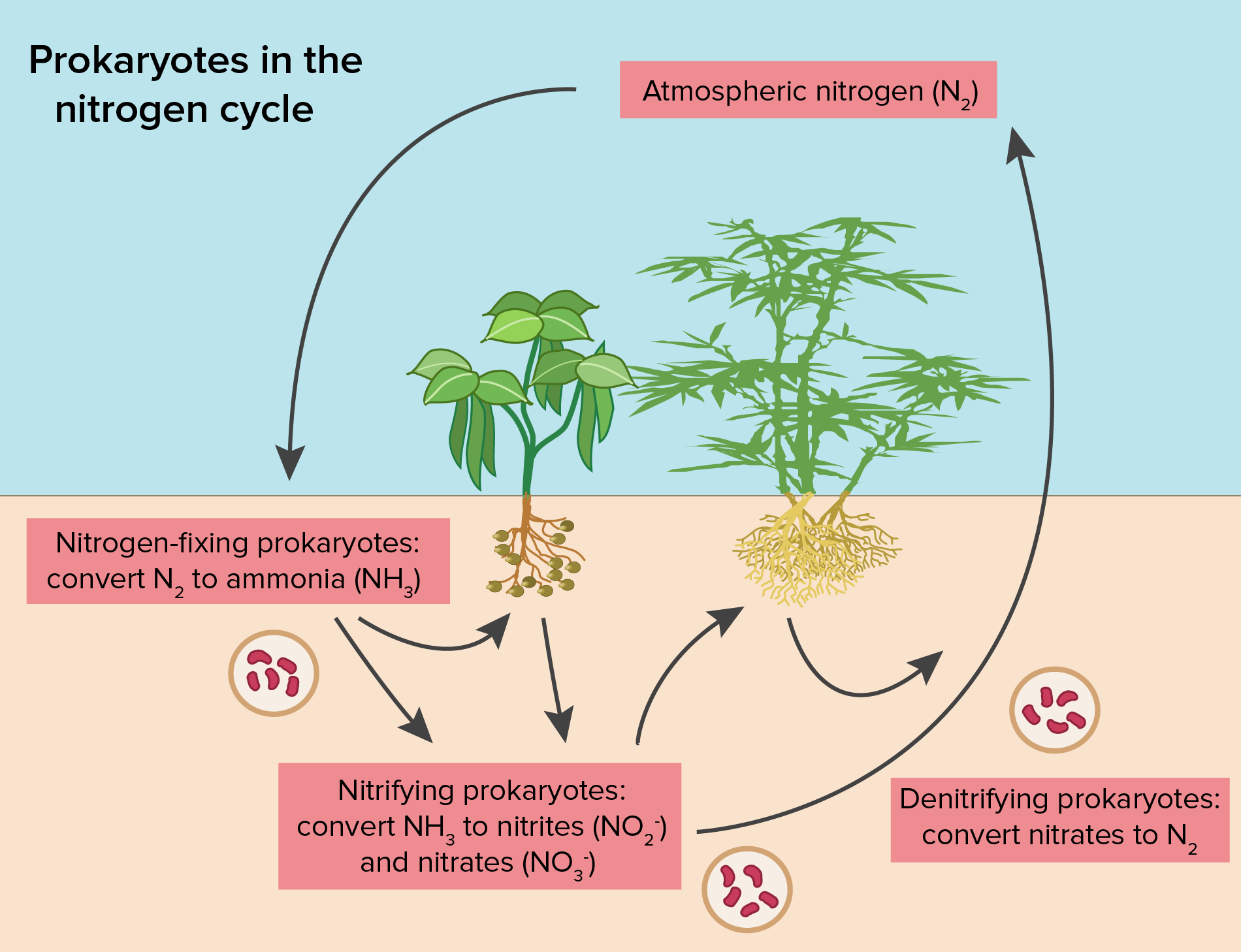
The Nitrogen Cycle Article Ecology Khan Academy
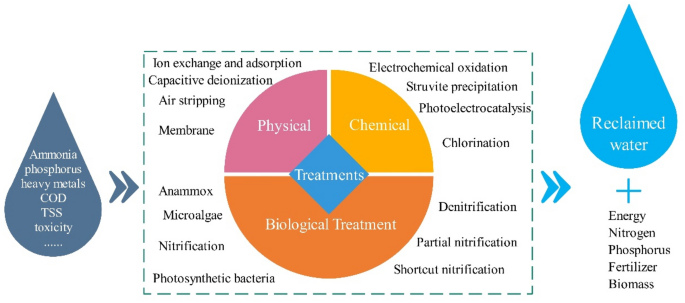
New Progress Of Ammonia Recovery During Ammonia Nitrogen Removal From Various Wastewaters Springerlink

Highly Efficient Electrochemical Reduction Of Nitrogen To Ammonia On Surface Termination Modified Ti3c2tx Mxene Nanosheets Acs Nano
New Progress Of Ammonia Recovery During Ammonia Nitrogen Removal From Various Wastewaters Springerlink

The Nitrogen Cycle Nitrifying Bacteria Use Oxygen And Alkalinity To Download Scientific Diagram

Recovery And Applications Of Ammoniacal Nitrogen From Nitrogen Loaded Residual Streams A Review Sciencedirect